
Video lecture for Atmospheres section
Now that we have seen what determines if a planet has an atmosphere or not, let's look at how atmospheres behave and how they affect the conditions on the surface of a planet. Only three things are needed to describe how a gas will behave: temperature, pressure, and density. Temperature and density have already been discussed, although for gases it is usually easier to use "number density" as in "number of particles per volume" instead of the usual mass density. Let's take a closer look at pressure.
Pressure is the amount of force exerted on a surface per unit area such as a number of newtons per square meter (or pounds of force per square inch). In the metric system, the unit of pressure is the pascal (Pa). This pressure is supplied by all of the particles in an atmosphere colliding into each other. At the Earth's surface there are approximately 25 million trillion (2.5 x 1019) molecules in every cubic centimeter moving about at speeds of hundreds of meters per second, so yes, they are going to bump into each other!
At the Earth's surface at sea level there is about 100 kilopascals of pressure exerted all over your body. For planet atmospheres, scientists will usually use a unit called a "bar" equal to 100 kilopascals, so the Earth's surface pressure at sea level is about 1 bar. That pressure is equivalent to about 1 kilogram pressing down in one Earth gravity on every square centimeter. If an adult pinky is about 1 centimeter in width (more or less), your body has quite a lot of square centimeters of surface area. So why do you not feel all that weight pushing you down at the Earth's surface? As described in the figure below, the air pressure pushes in all directions equally and the pressure of the fluid in your body pushing outward balances the air pressure.

Given the three parameters of temperature, density, and pressure, how the gas behaves is described by the equation of state. Most gases will obey a simple equation of state called the ideal gas law in which a doubling of the temperature or a doubling of the (number) density leads to a doubling of the pressure. For example, if you blow twice as much air into a balloon, the gas inside the balloon will push outward with twice as much pressure and the elastic material will expand until a new pressure balance is reached with the outside air pressure. Heating the air inside a hot air balloon increases the pressure inside the fabric enclosure so the balloon fabric that started out laid out all flat on the ground is now puffed into a round shape. (This also explains why your car's manual will tell you to measure the air pressure of your tires when they are cold, so you if you have been driving for a while, you will need to wait several minutes at least for the air inside the tire to cool off to get an accurate tire pressure reading.)
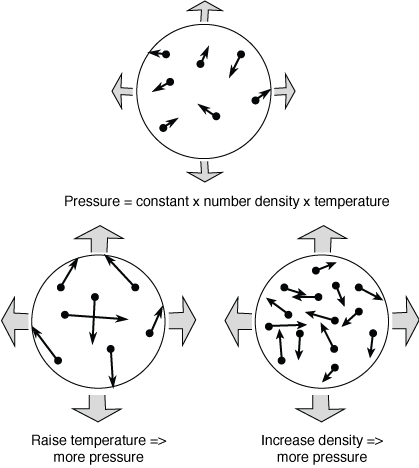
Let's continue with the hot air balloon example to make another important point. Once the balloon fabric is all puffed up, raising the temperature further inside the balloon will cause the air inside to flow out of the hole in the bottom of the balloon and density inside will drop. At the same pressure, less dense things will float upward (Archimedes' principle)---the hot air balloon will rise up off the ground. At a given pressure, cooler air is more dense than hotter air so the cooler air will sink. In an atmosphere, rising warmer air and sinking cooler air can transfer heat energy from a hotter surface to a cooler upper layer of the atmosphere in a process called convection that will be covered in more detail later.
Gravity pulls downward/inward on the molecules in an atmosphere but atmospheres remain "puffy" because of the moving gas particles supply pressure upward/outward. An atmosphere will not get puffier or shrink if the outward thermal pressure of the gases is balances by the inward gravity compression. This balance between pressure and gravity is called hydrostatic equilibrium. In the interior of planets, the resistance of the solid or liquid material supplies the pressure. In an atmosphere, the moving gases supply the pressure. If the Earth was at the distance of Pluto from the Sun, the nitrogen, oxygen, water, etc. in our air would freeze out and gravity would cause it all to collect on the surface about 12 meters thick. At our warmer position, these materials are in a gaseous state and make a layer 100 kilometers thick. [More accurately, our atmosphere extends out even further, beyond where the Space Shuttle can reach and where the International Space Station is but the air pressure is extremely small so 100 kilometers has been set as the "boundary" (fuzzy though it is) where space begins. Objects in low Earth orbit do feel a slight drag though and therefore need to be periodically boosted back up to their original orbit or they will spiral downward and burn up in our atmosphere.]


Lower layers of the atmosphere feel greater gravity compression from all of the material in the layers above pushing down on them. Therefore, they exert greater pressure to keep the balance.

![]() Go back to previous section --
Go back to previous section --
![]() Go to next section
Go to next section
last updated: March 20, 2022