Video lecture for Atmospheres section
A planet's atmosphere helps shield a planet's surface from harsh radiation from the Sun and it moderates the amount of energy lost to space from the planet's interior. An atmosphere also makes it possible for liquid to exist on a planet's surface by supplying the pressure needed to keep the liquid from boiling away to space---life on the surface of a planet or moon requires an atmosphere. All of the planets started out with atmospheres of hydrogen and helium. The inner four planets (Mercury, Venus, Earth, and Mars) lost their original atmospheres. The atmospheres they have now are from gases released from their interiors, but Mercury and Mars have even lost most of their secondary atmospheres. The outer four planets (Jupiter, Saturn, Uranus, and Neptune) were able to keep their original atmospheres. They have very thick atmospheres with proportionally small solid cores while the the inner four planets have thin atmospheres with proportionally large solid parts.
The properties of each planet's atmosphere are summarized in the Planet Atmospheres table (will appear in a new window). Two key determinants in how thick a planet's atmosphere will be are the planet's escape velocity and the temperature of the atmosphere.
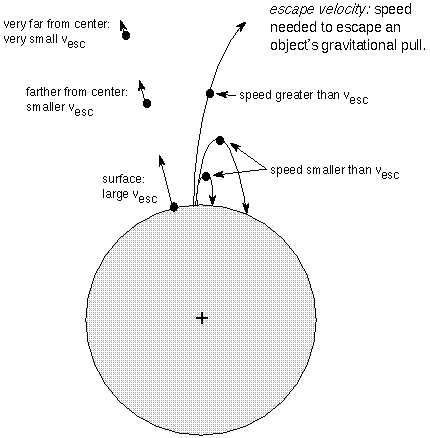
If you throw a rock up, it will rise up and then fall back down because of gravity. If you throw it up with a faster speed, it will rise higher before gravity brings it back down. If you throw it up fast enough it just escapes the gravity of the planet---the rock initially had a velocity equal to the escape velocity. The escape velocity is the initial velocity needed to escape a massive body's gravitational influence. In the Newton's Law of Gravity chapter the escape velocity is found to = Sqrt[(2G × (planet or moon mass))/distance)]. The distance is measured from the planet or moon's center.

Since the mass is in the top of the fraction, the escape velocity increases as the mass increases. A more massive planet will have stronger gravity and, therefore, a higher escape velocity. Also, because the distance is in the bottom of the fraction, the escape velocity decreases as the distance increases. The escape velocity is lower at greater heights above the planet's surface. The planet's gravity has a weaker hold on the molecules at the top of the atmosphere than those close to the surface, so those high up molecules will be the first to ``evaporate away.''
Do not confuse the distance from the planet's center with the planet's distance from the Sun. The escape velocity does NOT depend on how far the planet is from the Sun. You would use the Sun's distance only if you wanted to calculate the escape velocity from the Sun. In the same way, a moon's escape velocity does NOT depend on how far it is from the planet it orbits.

The particles in a hotter gas are moving quicker than those in a cooler gas of the same type. Using Newton's laws of motion, the relation between the speeds of the molecules and their temperature is found to be temperature = (gas molecule mass)×(average gas molecule speed)2 / (3k), where k is a universal constant of nature called the ``Boltzmann constant''. Gas molecules of the same type and at the same temperature will have a spread of speeds---some moving quickly, some moving slower---so use the average speed.
If you switch the temperature and velocity, you can derive the average gas molecule velocity = Sqrt[(3k × temperature/(molecule mass))]. Remember that the mass here is the tiny mass of the gas particle, not the planet's mass. Since the mass is in the bottom of the fraction, the more massive gas molecules will move slower on average than the lighter gas molecules. For example, carbon dioxide molecules move slower on average than hydrogen molecules at the same temperature. Because massive gas molecules move slower, planets with weaker gravity (e.g., the terrestrial planets) will tend to have atmospheres made of just massive molecules. The lighter molecules like hydrogen and helium will have escaped.

The dependence of the average speed of the gas molecules on their mass also explains the compositional structure observed in planet atmospheres. Since the distance a gas molecule can move away from the surface of a planet depends only on how fast it is moving and the planet's gravity, the lighter gas molecules can be found both close to the surface and far above it where the gravity is weaker. The gas molecules high up in the atmosphere are most likely to escape. The massive gas molecules will stay close to the planet surface. For example, the Earth's atmosphere is made of nitrogen, oxygen, and water molecules and argon atoms near the surface but at the upper-most heights, hydrogen and helium predominate.

Whereas the process described above leads to evaporation molecule by molecule, another type of atmospheric loss from heating happens when the atmosphere absorbs ultraviolet light, warms up and expands upward leading to a planetary wind flowing outward to space. Planets with a lot of hydrogen in their atmospheres are especially subject to this sort of atmospheric loss from heating. The very light hydrogen can bump heavier molecules and atoms outward in the planetary wind.

The effects of gravity and temperature work opposite to each other. A higher temperature tries to dissipate an atmosphere while higher gravity tries to retain an atmosphere. If the particle's average speed is close to the escape velocity, then those type of gas particles will not remain for billions of years. The general rule is: if the average gas molecule speed for a type of gas is less than than 0.2×(the escape velocity), then more than 1/2 of that type of gas will be left after one billion years. If the average speed is greater than that critical value, then more than 1/2 of that type of gas will be gone after one billion years. A flowchart of this is given on the escaping atmosphere page.
Because the jovian planets are massive and cold, they have THICK atmospheres of hydrogen and helium. The terrestrial planets are small in mass and warm, so they have thin atmospheres made of heavier molecules like carbon dioxide or nitrogen.
Test and improve your understanding of these concepts with the UNL Astronomy Education program's Atmospheric Retention module (link will appear in a new window). Note that it does use some simplifications but it provides a nice way to show the roles of temperature and escape velocity in determining how thick a planet's atmosphere will be.
The processes described above occur from the heating of the atoms and molecules in an atmosphere to the point where they can escape the planet's gravity. They are called thermal processes. Other ways involve the presence or lack of a magnetic field and asteroid or comet impacts. Ions are atoms that have an extra charge (usually by losing an electron). Ions will spiral around magnetic field lines so a planet's magnetic field (discussed more in a later section) will have a lot of ions trapped in it. When a fast-moving hydrogen ion (a proton) bumps into a neutral atom it can steal an electron to become a neutral atom that is not trapped by the magnetic field and it escapes the planet's gravity. This is called charge-exchange. Some of the magnetic field lines are so wide that they get stretched out by the high-speed stream of ions from the Sun called solar wind. The stretched out lines do not loop back and just open out into interplanetary space. Ions spiraling around these open magnetic field lines can escape along those lines in what is called a polar wind.

If a planet does not have a magnetic field (for reasons described later), the solar wind can strip an atmosphere through a process called sputtering. Without a magnetic field, the solar wind is able to hit the planet's atmosphere directly. The high-energy solar wind ions can accelerate atmosphere particles at high altitudes to great enough speeds to escape. An additional way of atmosphere escape called photodissociation occurs when high-energy sunlight (e.g., ultraviolet or x-rays) hits high-altitude molecules in the planet's atmosphere and breaks them apart into individual atoms or smaller molecules. These smaller particles have the same temperature as the larger molecules and, therefore, as described above, will move at faster speeds, possibly fast enough to escape.
The processes described so far in this section work particle to particle and work over long time periods as the atmosphere leaks away particle by particle. In contrast impacts by comets or asteroids can inject a huge amount of energy very quickly when the projectile vaporizes upon impact. The expanding plume of hot gas drives off the air above the impact site, with the larger the impact energy, the wider is the cone of air that is removed above the impact site. The impact removal process was probably particularly effective for Mars (being so close to the asteroid belt) and the large moons of Jupiter (so close to Jupiter's strong gravity that attracts numerous comets and asteroids).
![]() Go back to previous section --
Go back to previous section --
![]() Go to next section
Go to next section
last updated: March 20, 2022