This material (including images) is copyrighted!. See my copyright notice for fair use practices.
Now that you know what kinds of stars would be good to explore further and what criteria should be used for distinguishing lifeforms from other physical processes, let us hone in on the right kind of planet to support life. Unfortunately, our information about life is limited to one planet, Earth, so the Earth-bias is there. However, scientists do know of the basics of what life needs and what sort of conditions would probably destroy life. With these cautionary notes, let's move forward.

The habitable planet should have:
Liquid water is best for biochemical reactions
and could be very abundant but liquid methane and/or ethane, like what is found
on Saturn's moon Titan might work. Since liquid water dissolves other compounds better than liquid methane/ethane and biochemical
sort of reactions work better in liquid water than liquid methane/ethane, liquid
water will probably be a requirement for a habitable planet. Water is liquid at a wide temperature range. Bio-chemical reactions will not happen in solids and they would be very inefficient in a gas. Water is liquid at a higher temperature than methane, ethane, and ammonia so chemical reactions will happen more quickly in the liquid water than in the other liquids. Also, frozen water floats! The hydrogen bonds of water make water less dense when it freezes. The frozen water ice could form a protective layer insulating the liquid water below it. The other types of liquids sink when they freeze and could lead to a runaway freezing process where all of the liquid freezes. Finally, water in
some form (mostly either gas or solid) is actually quite abundant in the Galaxy
so we are not limiting ourselves too much with the water bias. The liquid milieu
is needed to mix...
Carbon will probably be the base of life because its great versatility to form compounds with other elements and even with itself. Carbon is more likely to share its electrons with other atoms rather than donate its electrons to other atoms or steal electrons from other atoms. Carbon has the highest degree of "catenation" (ability to form chemical bonds to itself) of all the elements. There are far more types of organic compounds (molecules containing carbon and usually also hydrogen) known than all the other types of compounds combined. On a planet with carbon-based life and life using carbon's closest competitor, silicon, as a base, the carbon-based chemical reactions would be far more efficient than the silicon-based ones, so the carbon-based life would quickly overrun any silicon-based life present on the planet. For more on silicon as a base for life, see the Scientific American "Ask the Experts" answer written by Raymond Dessy (link appears in a new window).

On planets or moons without an atmosphere and/or that are far from their parent star, it may be possible to have life existing below the surface if the planet or moon have a planetary heating source. An example of this would be Jupiter's moon Europa. It has a water ice crust and a liquid water ocean below and is kept warm despite its great distance from the Sun because of tidal heating from Jupiter's large gravity. Explore the Planetary Habitability Laboratory website for more about the research into what makes a habitable planet and the list of exoplanet websites in the Solar System Fluff chapter for what we're doing to find habitable exoplanets.
Although the rest of this chapter focuses on water-based life, the existence of methane lakes and rivers on Titan in our solar system compels us to consider life that could use liquid methane as the solvent to mix the organic chemicals about in its biochemistry. Another reason to consider methane-based life is that there are likely more very cold places where liquid methane could exist in our galaxy (and others as well) than liquid water places. For example, methane-based life could exist on exoplanets much further out from the very abundant cool K and M stars than what water-based life would be able to withstand---the habitable zone for methane-based life would be further out than that for water-based life. Planets in a "methane habitable zone" of a cool K and M star would not have their rotations tidally locked to the star.
With regard to Titan, methane-based life would have a ready supply of food from the acetylene and ethane raining down to the surface as a result of the photochemistry of ultraviolet light in sunlight breaking apart the methane vapor in Titan's atmosphere. Using the hydrogen also present in Titan's atmosphere, methanogens (organisms producing methane) would combine hydrogen with acetylene and ethane (and other hydrocarbons) to produce methane and energy. Titan life would need to develop special enzymes to extract oxygen from the water-ice rocks but the other essential elements such as carbon, hydrogen, and nitrogen would be easy to come by in the environment of Titan's surface. See Chris McKay's talk in the Silicon Valley Astronomers Lecture Series for more on the possibilities of life on Titan.
A recent study of the reflectivity of the surfaces of the lakes on Titan suggests that frozen methane ice might be able to float if the conditions are just right: if the temperatures were in a narrow range just below the freezing point of methane (like in Titan's winters) and if the ice were composed of at least 5% nitrogen gas that is quite abundant in Titan's atmosphere. However, if the temperature drops by a few more degrees, the ice will sink. An atmosphere of different composition on a cold exoplanet might get the frozen methane to float with a different temperature range. One last thing to note about methane-based life on a cold world is that the metabolic life cycles of an organism could be measured in time intervals of tens of thousands of years instead of the hours or days we are used on Earth, making it even more difficult to detect the metabolic processes. Also, it is very likely that any methane-based life is going to be microbial only. A complex, multi-cellular intelligent organism is much more likely to use oxygen in its metabolism with water as its liquid medium of choice.
While it may be possible for life to exist on a planet or moon below its surface, we will not be able to detect its presence from a great distance away (e.g., if it is in another star system beyond our solar system). In our fastest rocket-propelled spacecraft, it would take us over 70,000 years to travel to the next star system (Alpha Centauri). The type of inhabited planet we will be able to detect outside of our solar system is life that has changed the chemistry of the planet's atmosphere, i.e., the life will have to be on the surface. By analyzing the spectrum of the planet's atmosphere, we may be able to detect bio-markers---spectral signatures of certain compounds in certain proportions that could not be produced by non-biological processes. Bio-markers are also "biosignatures"
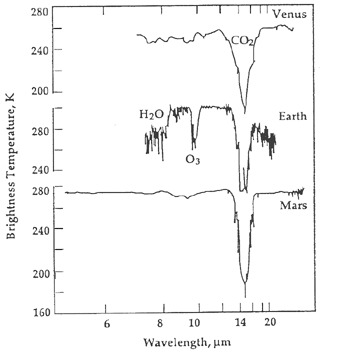
Spectral lines from water would say that a planet has a vital ingredient for life but it does not mean that life is present. If oxygen, particularly ozone (a molecule of three oxygen atoms), is found in the atmosphere, then it would be very likely that life is indeed on the planet. Recall from the solar system chapter that molecular oxygen quickly disappears if it is not continually replenished by the photosynthesis process of plants and cyanobacteria. However, it is conceivably possible for a few non-biological processes to create an atmosphere rich in molecular oxygen and ozone. For example, on a planet with a runaway greenhouse effect, ultraviolet light from the star could break apart the molecules of carbon dioxide and water to make a significant amount of molecular oxygen and ozone. This is especially true for stars that produce proportionally more short-wavelength ultraviolet (far UV) light than long-wavelength ultraviolet (near UV) light. Many red dwarf stars, including the nearby ones such as Gliese 832 with super-Earth-size planets orbiting them, produce a lot more far UV than near UV, so a strong oxygen spectral line could be a "false-positive" sign of life.
Molecular oxygen does not produce absorption lines in the preferred infrared band that will be used by the upcoming James Webb Space Telescope and the proposed Terrestrial Planet Finder mission. Ozone does. If we take into account the ultraviolet environment of the exoplanet, then ozone existing along with nitrous oxide and methane in particular ratios with carbon dioxide and water, all of which produce absorption lines in the infrared, would be strong evidence for an inhabited world. The ratios would need to be "off-kilter", not in chemical equilibrium, i.e., not in ratios made by normal geological processes. For such worlds found with these bio-markers, further modeling of what strange non-biological water cycles and volcanic activity very different from that found on Earth could produce the large amount of ozone would need to be done before we could definitively conclude that the exoplanet had life on it. It is a very big step to go from finding a planet that could support life to saying that the planet does support life!
One recent test of ozone bio-marker concept was when the Venus Express spacecraft pointed its spectrometer at Earth in August 2007 while the spacecraft was orbiting Venus 78 million kilometers from the Earth. The near-infrared spectra of the Earth is shown for two different observing sessions. Earth was just the size of a single pixel in its camera. The part of the Earth facing the Venus Express spacecraft is shown in the simulated image above the spectra.
An exoplanet will need to have enough oxygen (either as molecular oxygen or ozone) in its atmosphere for us to detect. If the history of an exoplanet's atmosphere is anything like ours, then life on the exoplanet's surface might not be detectable for a large fraction of the exoplanet's history. Photosynthetic life developed on the Earth at least 3.5 billion years ago (Gya) but it took another 1.2 billion years or so (i.e., 2.3 Gya) for the oxygen levels in the atmosphere to rise up to significant quantities because the oxygen was combining with land and ocean minerals (to make iron oxide and other oxides). Only after 1.2 billion years or so did the surface and ocean minerals get too saturated to suck up any more of the oxygen, allowing the oxygen to build up in our atmosphere.
One example of the research into how the spectrum of an exoplanet can change through time is shown in the figure below from Kaltenegger, et al's paper on the Earth's changing spectrum through time. The light gray curve is what an ultra-high resolution spectrometer would be able to see (the absorption lines are so numerous and close together that they merge into gray bands at the scale of the graph) and the thick black line is what an actual spectrometer with realistic resolution on the proposed Terrestrial Planet Finder mission would be able to see. This particular set of spectra is for a planet without any clouds in the way. See their paper for how clouds in the atmosphere would affect the spectrum and also for the spectrum in the visible and near-infrared bands.
Could life exist on a planet without oxygen? Yes. Photosynthesis might be able to use another element such as sulfur instead of oxygen. The planet's life might use another liquid besides water. Maybe the planet's life would use a different element besides carbon as its base (such as silicon). The first missions that will hunt for life beyond the Earth will focus on biochemical processes that we are more familiar with (carbon-based life using liquid water) because it makes sense to start with what we know (or think we know) and then branch out to finding more exotic life after we have had some practice with the "ordinary" life. Detecting methane-based life on a cold world like Titan would require a lander to scoop up the organics in the soil to see if there are increased amounts of oxygen in the organics because the organisms would be scavenging the oxygen from the water-ice rocks.
Arney and Schwieterman gave an informative webinar on exoplanet biosignatures in November 2016 that is worth viewing to find out more about biosignatures in exoplanet atmospheres, what the Earth's spectrum would have looked like with a thick orange haze layer in the Archean eon (3.8 to 2.5 Gya), and potential "false positive" signals for life and how to avoid them. They focus on the use of oxygen, ozone, and methane as biosignatures.
![]() Go back to previous section --
Go back to previous section --
![]() Go to next section
Go to next section
last updated: July 1, 2022