Video lecture for this chapter
Close examination of the spectra from the Sun and other stars reveals that the rainbow of colors has many dark lines in it, called absorption lines. They are produced when cooler thin gas absorbs certain colors of light produced by hotter dense objects, so for stars, the cooler, low-density upper layers absorb certain colors produced by the deeper, denser layers. The cool thin gas is thin enough that most of the light can pass right through it without hitting any atoms or molecules (e.g., as thin as the upper layers of the Earth's atmosphere many tens of kilometers above the Earth's surface). You can also see them in the reflected light spectrum from planets. Some of the colors in the sunlight reflecting off the planets are absorbed by the molecules on the planet's surface or in its atmosphere.

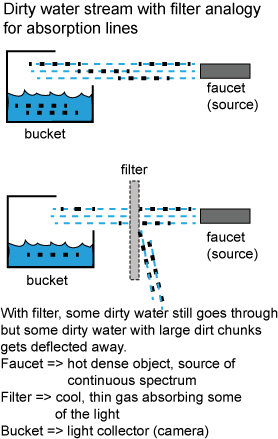
The process in making absorption lines is analogous to putting a filter in a narrow stream of dirty water that is collecting in a bucket. Without the filter, all of the dirty water and dirt chunks collect in the bucket. With the filter, some of the dirty water and the dirt chunks get deflected away from the bucket, so not as much of the dirty water and dirt chunks make it into the bucket. In the analogy, the source of the continuous spectrum light is like the faucet spewing out the dirty water, the cool thin gas is like the filter, and the bucket is like the camera or telescope. What is not in the analogy is the spectrometer that takes the collected light and spreads it out into its constituent colors.
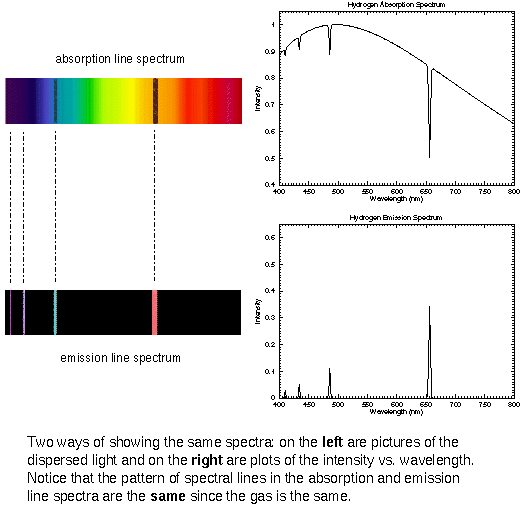
The spectra of hot, thin (low density) gas clouds are a series of bright lines called emission lines. In both of these types of spectra you see spectral features at certain, discrete wavelengths (or colors) and no where else.
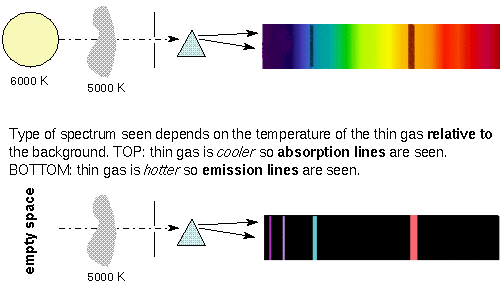
The type of spectrum you see depends on the temperature of the thin gas. If the thin gas is cooler than the thermal source in the background, you see absorption lines. Since the spectra of stars show absorption lines, it tells you that the density and temperature of the upper layers of a star is lower than the deeper layers. In a few cases you can see emission lines on top of the thermal spectrum. This is produced by thin gas that is hotter than the thermal source in the background. Unlike the case for absorption lines, though, the production of emission lines does NOT require a thermal source be in the background. The spectrum of a hydrogen-emission nebula (``nebula'' = gas or dust cloud) is just a series of emission lines without any thermal spectrum because there are no stars visible behind the hot nebula. Some objects produce spectra that is a combination of a thermal spectrum, emission lines, and absorption lines simultaneously!
What is very useful about discrete spectra is that the pattern of lines you see depends on the chemical composition of the thin gas. Each element or molecule produces a distinct pattern of lines---each element or molecule has a ``fingerprint'' you can use to identify it (like a UPC barcode or QR code). This allows you to remotely determine what stars, planets, nebulae, etc. are made of!
The composition canNOT be found from just one line because one element may have one spectral line at the same wavelength as another element's spectral line. However, an element's pattern of lines is unique. Using a single line to identify a gas would be like identifying the name of someone using just one letter of their name---many people will have that same letter in their name, but the pattern of letters (which letters and how they are arranged) is unique to that one person. Of course, stars, planets, nebulae, etc. are made of more than one type of material, so you see the discrete spectra of many elements and molecules superimposed on each other---all of the spectral lines add together. An experienced astronomer can disentangle all the different patterns and sort out the elements and molecules (but it does take time!).
A couple of nice interactives to try matching spectral lines to chemical composition are
To create your own spectra (graphs or tables), use the NIST Atomic Spectra Database Lines Form (from the National Institute of Standards and Technology).
![]() Go back to previous section --
Go back to previous section --
![]() Go to next section
Go to next section
last updated: January 13, 2022